10/13/2015
Cyanobacterial Alkanes: Today’s Bacterial Antifreeze, Tomorrow’s Fuel
A promising biofuel molecule helps the photosynthetic bacteria that naturally produce it tolerate cold temperatures.
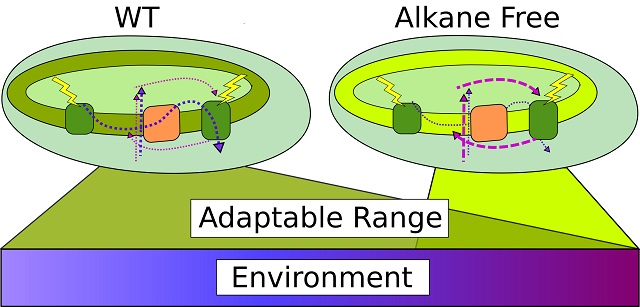
Cyanobacteria produce alkanes that modulate the balance between different energy production pathways. This feature allows these photosynthetic bacteria to thrive across a range of environmental temperatures.
Image modified courtesy Berla, B., et al. 2015. “Cyanobacterial Alkanes Modulate Photosynthetic Electron Flow to Assist Growth under Cold Stress,” Scientific Reports 5, 14894 via CC BY 4.0.
The Science
Cyanobacteria, photosynthetic microorganisms, contain a unique and universal pathway that converts fatty acids to alkanes, a promising biofuel candidate. Recent spectroscopic and modeling studies illuminated how the produced alkanes allow cyanobacteria to adjust photosynthetic activity to tolerate cold temperatures.
The Impact
Understanding the natural function of alkanes in cyanobacteria may lead to production of these molecules as biofuels. These bacteria-produced alkanes are excellent fuel candidates because they have high-energy content and are highly compatible with existing infrastructure for petroleum-based fuel distribution and use.
Summary
Cyanobacteria are photosynthetic bacteria that, like plants, consume carbon dioxide and produce oxygen through photosynthesis. All cyanobacterial membranes contain diesel-range C15-C19 hydrocarbons in high concentration and the production pathways for these metabolites are exclusive to cyanobacteria. In this study, the model cyanobacterium, Synechocystis sp. PCC 6803, was modified to produce no alkanes, and the resulting strain grew poorly at low temperatures. To understand the growth defect, the researchers assessed the redox kinetics of how cyanobacteria convert solar energy into chemical energy in the form of adenosine triphosphate (ATP) and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). ATP and NADPH are produced using a linear and a cyclic pathway with the pigment-protein complex, photosystem I (PSI), as the hub to both. The modified strain made greater use of the cyclic pathway, which raises the ATP:NADPH ratio, especially at low temperature. This use helps to balance reductant requirements and maintain the redox poise of the electron transport chain. While previous theories held that the cyclic pathway was used in a fixed ratio to the linear pathway, the researchers demonstrated that the cyclic pathway responds dynamically to the environment and that alkanes play a role in this response. Flux balance computational analysis showed that an intermediate use of the cyclic pathway (circa one-fourth that of the linear pathway) maximized growth as well. From this analysis, the team concluded that the lack of membrane alkanes required greater use of the cyclic pathway, presumably to maintain redox poise. In turn, such an increase compromises growth by activating energy-inefficient pathways. This study highlights the unique and universal role of medium-chain hydrocarbons in cyanobacteria: they regulate redox balance and reductant partitioning in these photosynthetic cells under stress.
Principal Investigator(s)
Himadri Pakrasi
Washington University in St. Louis
[email protected]
Related Links
Funding
This work was funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Biological Systems Science Division, Genomic Science Program.
References
Berla, B., R. Saha, C. Maranas, and H. Pakrasi. 2015. “Cyanobacterial Alkanes Modulate Photosynthetic Electron Flow to Assist Growth under Cold Stress,” Scientific Reports 5, 14894. DOI: 10.1038/srep14894.